In The News
Pain Medicine News, with support from Johnson & Johnson Consumer Inc., has issued a Special Report on the role of OTC analgesics in acute pain management when considering opioid-sparing approaches, with contributions from healthcare professionals on the front lines. The Special Report, though not exhaustive, includes case studies, real-world clinical scenarios, a review of current guidelines, and efficacy and safety profiles of OTC pain relievers.

The US Food and Drug Administration (FDA) recently required an update to the Drug Facts labeling of all adult, children’s, and infants’ non-aspirin over-the-counter (OTC) nonsteroidal anti-inflammatory drugs (NSAIDs), for example, Motrin®, Advil®, and Aleve® products.
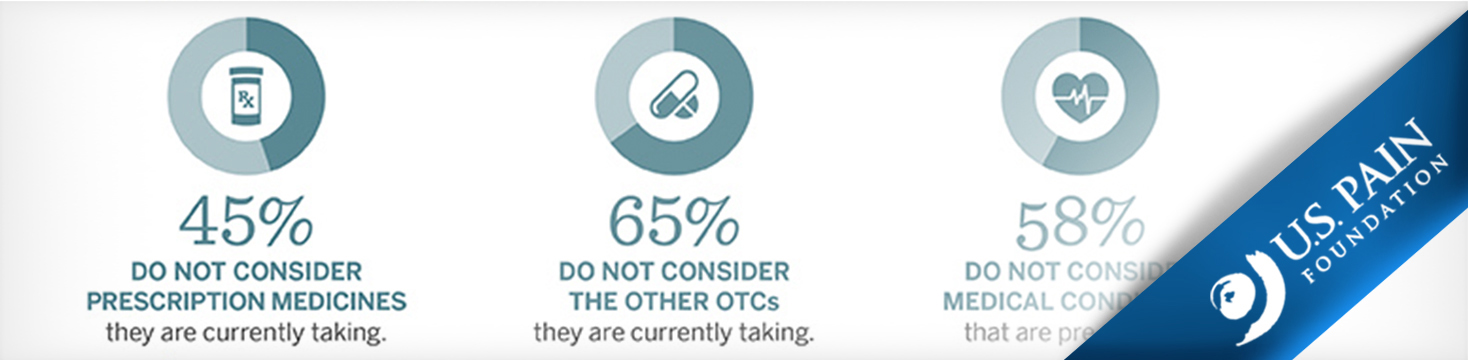
A recent survey1 conducted by the US Pain Foundation, with support from Johnson & Johnson Consumer Inc., found that 97% of Americans feel confident when choosing which type of over-the-counter (OTC) pain medicines to take. However, the survey also revealed that many consumers disregard critical safety factors—such as their age, health conditions, and other medicines they are currently taking—when choosing an OTC pain reliever.
Related news
March 6, 2017
Study, resources on OTC Pain Relievers
– The American Nurse
August 29, 2016
Press Release: 1 in 5 Americans do not consider any key safety factors when choosing OTC pain relievers
